The domain within your query sequence starts at position 5271 and ends at position 5313; the E-value for the EGF_CA domain shown below is 3.51e-10.
DIDECKDGTHQCRYNQICENTRGSYRCACPRGYRSQGVGRPCI
EGF_CACalcium-binding EGF-like domain |
|---|
| SMART accession number: | SM00179 |
|---|---|
| Description: | - |
| Interpro abstract (IPR001881): | A sequence of about forty amino-acid residues found in epidermal growth factor (EGF) has been shown [ (PUBMED:2288911) (PUBMED:6334307) (PUBMED:3534958) (PUBMED:6607417) (PUBMED:3282918) ] to be present in a large number of membrane-bound and extracellular, mostly animal, proteins. Many of these proteins require calcium for their biological function and a calcium-binding site has been found at the N terminus of some EGF-like domains [ (PUBMED:1527084) ]. Calcium-binding may be crucial for numerous protein-protein interactions. For human coagulation factor IX it has been shown [ (PUBMED:7606779) ] that the calcium-ligands form a pentagonal bipyramid. The first, third and fourth conserved negatively charged or polar residues are side chain ligands. The latter is possibly hydroxylated (see aspartic acid and asparagine hydroxylation site) [ (PUBMED:1527084) ]. A conserved aromatic residue, as well as the second conserved negative residue, are thought to be involved in stabilising the calcium-binding site. As in non-calcium binding EGF-like domains, there are six conserved cysteines and the structure of both types is very similar as calcium-binding induces only strictly local structural changes [ (PUBMED:1527084) ].
|
| GO function: | calcium ion binding (GO:0005509) |
| Family alignment: |
There are 282922 EGF_CA domains in 60844 proteins in SMART's nrdb database.
Click on the following links for more information.
- Evolution (species in which this domain is found)
-
Taxonomic distribution of proteins containing EGF_CA domain.
This tree includes only several representative species. The complete taxonomic breakdown of all proteins with EGF_CA domain is also avaliable.
Click on the protein counts, or double click on taxonomic names to display all proteins containing EGF_CA domain in the selected taxonomic class.
- Disease (disease genes where sequence variants are found in this domain)
-
SwissProt sequences and OMIM curated human diseases associated with missense mutations within the EGF_CA domain.
Protein Disease Fibrillin-2 (P35556) (SMART) OMIM:121050: Contractural arachnodactyly, congenital Vitamin K-dependent protein S (P07225) (SMART) OMIM:176880: Protein S deficiency E-selectin (P16581) (SMART) OMIM:131210: {Atherosclerosis, susceptibility to} Delta-like protein 3 (Q9NYJ7) (SMART) OMIM:602768: Spondylocostal dysostosis, autosomal recessive, 1
OMIM:277300:Fibrillin-1 (P35555) (SMART) OMIM:134797: Marfan syndrome
OMIM:154700: Shprintzen-Goldberg syndrome
OMIM:182212: Ectopia lentis, familial ; MASS syndrome
OMIM:604308:Coagulation factor IX (P00740) (SMART) OMIM:306900: Hemophilia B ; Warfarin sensitivity Low-density lipoprotein receptor (P01130) (SMART) OMIM:143890: Hypercholesterolemia, familial Vitamin K-dependent protein C (P04070) (SMART) OMIM:176860: Thrombophilia due to protein C deficiency ; Purpura fulminans, neonatal Thyroid peroxidase (P07202) (SMART) OMIM:274500: Thyroid iodine peroxidase deficiency ; Goiter, congenital ; Hypothyroidism, congenital EGF-containing fibulin-like extracellular matrix protein 1 (Q12805) (SMART) OMIM:601548: Doyne honeycomb degeneration of retina
OMIM:126600:
OMIM:126600: Doyne honeycomb retinal dystrophyCoagulation factor VII (P08709) (SMART) OMIM:227500: Factor VII deficiency ; {Myocardial infarction, decreased susceptibility to} - Metabolism (metabolic pathways involving proteins which contain this domain)
-
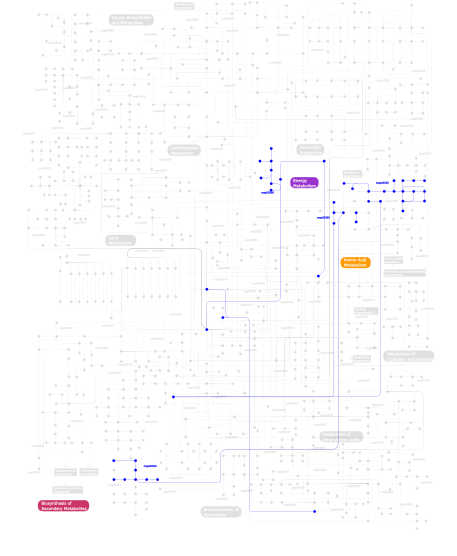
Click the image to view the interactive version of the map in iPath% proteins involved KEGG pathway ID Description 19.18 map04610 Complement and coagulation cascades 17.81 map04330 Notch signaling pathway 7.40 map04320 Dorso-ventral axis formation 6.85 map04510 Focal adhesion 4.93 map04360 Axon guidance 4.66 map04512 ECM-receptor interaction 4.66 map04350 TGF-beta signaling pathway 3.01 map04060 Cytokine-cytokine receptor interaction 2.19 map05219 Bladder cancer 2.19 map05213 Endometrial cancer 2.19 map04010 MAPK signaling pathway 2.19 map05212 Pancreatic cancer 2.19 map05215 Prostate cancer 2.19 map04012 ErbB signaling pathway 2.19 map05214 Glioma 2.19 map04810 Regulation of actin cytoskeleton 2.19 map04540 Gap junction 2.19 map04340 Hedgehog signaling pathway 2.19 map05223 Non-small cell lung cancer 2.19 map05218 Melanoma 1.92 map05010 Alzheimer's disease 0.82 map04640 Hematopoietic cell lineage 0.82  map00350
map00350Tyrosine metabolism 0.55  map00680
map00680Methane metabolism 0.55  map00940
map00940Phenylpropanoid biosynthesis 0.55  map00360
map00360Phenylalanine metabolism This information is based on mapping of SMART genomic protein database to KEGG orthologous groups. Percentage points are related to the number of proteins with EGF_CA domain which could be assigned to a KEGG orthologous group, and not all proteins containing EGF_CA domain. Please note that proteins can be included in multiple pathways, ie. the numbers above will not always add up to 100%.
- Structure (3D structures containing this domain)
3D Structures of EGF_CA domains in PDB
PDB code Main view Title 1apo 
THREE-DIMENSIONAL STRUCTURE OF THE APO FORM OF THE N-TERMINAL EGF-LIKE MODULE OF BLOOD COAGULATION FACTOR X AS DETERMINED BY NMR SPECTROSCOPY AND SIMULATED FOLDING 1apq 
STRUCTURE OF THE EGF-LIKE MODULE OF HUMAN C1R, NMR, 19 STRUCTURES 1aut 
HUMAN ACTIVATED PROTEIN C 1bf9 
N-TERMINAL EGF-LIKE DOMAIN FROM HUMAN FACTOR VII, NMR, 23 STRUCTURES 1c5m 
STRUCTURAL BASIS FOR SELECTIVITY OF A SMALL MOLECULE, S1-BINDING, SUB-MICROMOLAR INHIBITOR OF UROKINASE TYPE PLASMINOGEN ACTIVATOR 1ccf 
HOW AN EPIDERMAL GROWTH FACTOR (EGF)-LIKE DOMAIN BINDS CALCIUM-HIGH RESOLUTION NMR STRUCTURE OF THE CALCIUM FORM OF THE NH2-TERMINAL EGF-LIKE DOMAIN IN COAGULATION FACTOR X 1dan 
COMPLEX OF ACTIVE SITE INHIBITED HUMAN BLOOD COAGULATION FACTOR VIIA WITH HUMAN RECOMBINANT SOLUBLE TISSUE FACTOR 1dva 
Crystal Structure of the Complex Between the Peptide Exosite Inhibitor E-76 and Coagulation Factor VIIA 1edm 
EPIDERMAL GROWTH FACTOR-LIKE DOMAIN FROM HUMAN FACTOR IX 1emn 
NMR STUDY OF A PAIR OF FIBRILLIN CA2+ BINDING EPIDERMAL GROWTH FACTOR-LIKE DOMAINS, MINIMIZED AVERAGE STRUCTURE 1emo 
NMR STUDY OF A PAIR OF FIBRILLIN CA2+ BINDING EPIDERMAL GROWTH FACTOR-LIKE DOMAINS, 22 STRUCTURES 1ezq 
CRYSTAL STRUCTURE OF HUMAN COAGULATION FACTOR XA COMPLEXED WITH RPR128515 1f0r 
CRYSTAL STRUCTURE OF HUMAN COAGULATION FACTOR XA COMPLEXED WITH RPR208815 1f0s 
Crystal Structure of Human Coagulation Factor XA Complexed with RPR208707 1f7e 
THE FIRST EGF-LIKE DOMAIN FROM HUMAN BLOOD COAGULATION FVII, NMR, 20 STRUCTURES 1f7m 
THE FIRST EGF-LIKE DOMAIN FROM HUMAN BLOOD COAGULATION FVII, NMR, MINIMIZED AVERAGE STRUCTURE 1fak 
HUMAN TISSUE FACTOR COMPLEXED WITH COAGULATION FACTOR VIIA INHIBITED WITH A BPTI-MUTANT 1fax 
COAGULATION FACTOR XA INHIBITOR COMPLEX 1ff7 
THE FIRST EGF-LIKE DOMAIN FROM HUMAN BLOOD COAGULATION FVII (FUCOSYLATED AT SER-60), NMR, 20 STRUCTURES 1ffm 
THE FIRST EGF-LIKE DOMAIN FROM HUMAN BLOOD COAGULATION FVII (FUCOSYLATED AT SER-60), NMR, MINIMIZED AVERAGE STRUCTURE 1g2l 
FACTOR XA INHIBITOR COMPLEX 1g2m 
FACTOR XA INHIBITOR COMPLEX 1hj7 
NMR study of a pair of LDL receptor Ca2+ binding epidermal growth factor-like domains, 20 structures 1hz8 
SOLUTION STRUCTURE AND BACKBONE DYNAMICS OF A CONCATEMER OF EGF-HOMOLOGY MODULES OF THE HUMAN LOW DENSITY LIPOPROTEIN RECEPTOR 1i0u 
SOLUTION STRUCTURE AND BACKBONE DYNAMICS OF A CONCATEMER OF EGF-HOMOLOGY MODULES OF THE HUMAN LOW DENSITY LIPOPROTEIN RECEPTOR 1ioe 
Human coagulation factor Xa in complex with M55532 1iqe 
Human coagulation factor Xa in complex with M55590 1iqf 
Human coagulation factor Xa in complex with M55165 1iqg 
Human coagulation factor Xa in complex with M55159 1iqh 
Human coagulation factor Xa in complex with M55143 1iqi 
Human coagulation factor Xa in complex with M55125 1iqj 
Human coagulation factor Xa in complex with M55124 1iqk 
Human coagulation factor Xa in complex with M55113 1iql 
Human coagulation factor Xa in complex with M54476 1iqm 
Human coagulation factor Xa in complex with M54471 1iqn 
Human coagulation factor Xa in complex with M55192 1ixa 
THE THREE-DIMENSIONAL STRUCTURE OF THE FIRST EGF-LIKE MODULE OF HUMAN FACTOR IX: COMPARISON WITH EGF AND TGF-A 1ksn 
Crystal Structure of Human Coagulation Factor XA Complexed with FXV673 1lmj 
NMR Study of the Fibrillin-1 cbEGF12-13 Pair of Ca2+ Binding Epidermal Growth Factor-like Domains 1lpg 
CRYSTAL STRUCTURE OF FXA IN COMPLEX WITH 79. 1lpk 
CRYSTAL STRUCTURE OF FXA IN COMPLEX WITH 125. 1lpz 
CRYSTAL STRUCTURE OF FXA IN COMPLEX WITH 41. 1lqd 
CRYSTAL STRUCTURE OF FXA IN COMPLEX WITH 45. 1n7d 
Extracellular domain of the LDL receptor 1nfu 
CRYSTAL STRUCTURE OF HUMAN COAGULATION FACTOR XA COMPLEXED WITH RPR132747 1nfw 
CRYSTAL STRUCTURE OF HUMAN COAGULATION FACTOR XA COMPLEXED WITH RPR209685 1nfx 
CRYSTAL STRUCTURE OF HUMAN COAGULATION FACTOR XA COMPLEXED WITH RPR208944 1nfy 
CRYSTAL STRUCTURE OF HUMAN COAGULATION FACTOR XA COMPLEXED WITH RPR200095 1nt0 
Crystal structure of the CUB1-EGF-CUB2 region of MASP2 1nzi 
Crystal Structure of the CUB1-EGF Interaction Domain of Complement Protease C1s 1o5d 
Dissecting and Designing Inhibitor Selectivity Determinants at the S1 site Using an Artificial Ala190 Protease (Ala190 uPA) 1p0s 
Crystal Structure of Blood Coagulation Factor Xa in Complex with Ecotin M84R 1pfx 
PORCINE FACTOR IXA 1szb 
Crystal structure of the human MBL-associated protein 19 (MAp19) 1toz 
NMR structure of the human NOTCH-1 ligand binding region 1uzj 
Integrin binding cbEGF22-TB4-cbEGF33 fragment of human fibrillin-1, holo form. 1uzk 
Integrin binding cbEGF22-TB4-cbEGF33 fragment of human fibrillin-1, Ca bound to cbEGF23 domain only 1uzp 
Integrin binding cbEGF22-TB4-cbEGF33 fragment of human fibrillin-1, Sm bound form cbEGF23 domain only. 1uzq 
Integrin binding cbEGF22-TB4-cbEGF33 fragment of human fibrillin-1, apo form cbEGF23 domain only. 1w0y 
tf7a_3771 complex 1w2k 
tf7a_4380 complex 1whe 
COAGULATION FACTOR, NMR, 20 STRUCTURES 1whf 
COAGULATION FACTOR, NMR, 15 STRUCTURES 1wqv 
Human Factor Viia-Tissue Factor Complexed with propylsulfonamide-D-Thr-Met-p-aminobenzamidine 1wss 
Human Factor Viia-Tissue Factor in Complex with peprid mimetic inhibitor that has two charge groups in P2 and P4 1wtg 
Human Factor Viia-Tissue Factor Complexed with ethylsulfonamide-D-biphenylalanine-Gln-p-aminobenzamidine 1wu1 
Factor Xa in complex with the inhibitor 4-[(5-chloroindol-2-yl)sulfonyl]-2-(2-methylpropyl)-1-[[5-(pyridin-4-yl) pyrimidin-2-yl]carbonyl]piperazine 1wun 
Human Factor Viia-Tissue Factor Complexed with ethylsulfonamide-D-Trp-Gln-p-aminobenzamidine 1wv7 
Human Factor Viia-Tissue Factor Complexed with ethylsulfonamide-D-5-propoxy-Trp-Gln-p-aminobenzamidine 1x7a 
Porcine Factor IXa Complexed to 1-{3-[amino(imino)methyl]phenyl}-N-[4-(1H-benzimidazol-1-yl)-2-fluorophenyl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide 1xka 
FACTOR XA COMPLEXED WITH A SYNTHETIC INHIBITOR FX-2212A,(2S)-(3'-AMIDINO-3-BIPHENYLYL)-5-(4-PYRIDYLAMINO)PENTANOIC ACID 1xkb 
FACTOR XA COMPLEXED WITH A SYNTHETIC INHIBITOR FX-2212A,(2S)-(3'-AMIDINO-3-BIPHENYLYL)-5-(4-PYRIDYLAMINO)PENTANOIC ACID 1z6c 
Solution structure of an EGF pair (EGF34) from vitamin K-dependent protein S 1z6j 
Crystal Structure of a ternary complex of Factor VIIa/Tissue Factor/Pyrazinone Inhibitor 2a2q 
Complex of Active-site Inhibited Human Coagulation Factor VIIa with Human Soluble Tissue Factor in the Presence of Ca2+, Mg2+, Na+, and Zn2+ 2aei 
Crystal structure of a ternary complex of factor VIIa/tissue factor and 2-[[6-[3-(aminoiminomethyl)phenoxy]-3,5-difluro-4-[(1-methyl-3-phenylpropyl)amino]-2-pyridinyl]oxy]-benzoic acid 2aer 
Crystal Structure of Benzamidine-Factor VIIa/Soluble Tissue Factor complex. 2b7d 
Factor VIIa Inhibitors: Chemical Optimization, Preclinical Pharmacokinetics, Pharmacodynamics, and Efficacy in a Baboon Thrombosis Model 2b8o 
Crystal Structure of Glu-Gly-Arg-Chloromethyl Ketone-Factor VIIa/Soluble Tissue Factor Complex 2bo2 
EGF Domains 1,2,5 of human EMR2, a 7-TM immune system molecule, in complex with calcium. 2boh 
Crystal structure of factor Xa in complex with compound ""1"" 2bou 
EGF Domains 1,2,5 of human EMR2, a 7-TM immune system molecule, in complex with barium. 2box 
EGF Domains 1,2,5 of human EMR2, a 7-TM immune system molecule, in complex with strontium. 2c4f 
crystal structure of factor VII.stf complexed with pd0297121 2cji 
Crystal structure of a Human Factor Xa inhibitor complex 2ec9 
Crystal structure analysis of human Factor VIIa , Souluble tissue factor complexed with BCX-3607 2f9b 
Discovery of Novel Heterocyclic Factor VIIa Inhibitors 2fir 
Crystal structure of DFPR-VIIa/sTF 2flb 
Discovery of a Novel Hydroxy Pyrazole Based Factor IXa Inhibitor 2flr 
Novel 5-Azaindole Factor VIIa Inhibitors 2h9e 
Crystal Structure of FXa/selectide/NAPC2 ternary complex 2j2u 
CRYSTAL STRUCTURE OF A HUMAN FACTOR XA INHIBITOR COMPLEX 2j34 
CRYSTAL STRUCTURE OF A HUMAN FACTOR XA INHIBITOR COMPLEX 2j38 
CRYSTAL STRUCTURE OF A HUMAN FACTOR XA INHIBITOR COMPLEX 2j4i 
CRYSTAL STRUCTURE OF A HUMAN FACTOR XA INHIBITOR COMPLEX 2j94 
CRYSTAL STRUCTURE OF A HUMAN FACTOR XA INHIBITOR COMPLEX 2j95 
CRYSTAL STRUCTURE OF A HUMAN FACTOR XA INHIBITOR COMPLEX 2rqz 
Structure of sugar modified epidermal growth factor-like repeat 12 of mouse Notch-1 receptor 2rr0 
Structure of epidermal growth factor-like repeat 12 of mouse Notch-1 receptor 2rr2 
Structure of O-fucosylated epidermal growth factor-like repeat 12 of mouse Notch-1 receptor 2uwl 
Selective and Dual Action Orally Active Inhibitors of Thrombin and Factor Xa 2uwo 
Selective and Dual Action Orally Active Inhibitors of Thrombin and Factor Xa 2uwp 
Factor Xa inhibitor complex 2vh0 
Structure and property based design of factor Xa inhibitors:biaryl pyrrolidin-2-ones incorporating basic heterocyclic motifs 2vh6 
Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with biaryl P4 motifs 2vj2 
Human Jagged-1, domains DSL and EGFs1-3 2vj3 
Human Notch-1 EGFs 11-13 2w2m 
WT PCSK9-DELTAC BOUND TO WT EGF-A OF LDLR 2w2n 
WT PCSK9-deltaC bound to EGF-A H306Y mutant of LDLR 2w2o 
PCSK9-deltaC D374Y mutant bound to WT EGF-A of LDLR 2w2p 
PCSK9-deltaC D374A mutant bound to WT EGF-A of LDLR 2w2q 
PCSK9-deltaC D374H mutant bound to WT EGF-A of LDLR 2w86 
Crystal structure of fibrillin-1 domains cbEGF9hyb2cbEGF10, calcium saturated form 2wyg 
Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with monoaryl P4 motifs 2wyj 
Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with monoaryl P4 motifs 2y7x 
The discovery of potent and long-acting oral factor Xa inhibitors with tetrahydroisoquinoline and benzazepine P4 motifs 2y7z 
Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with aminoindane and phenylpyrrolidine P4 motifs 2y80 
Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with aminoindane and phenylpyrrolidine P4 motifs 2y81 
Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with aminoindane and phenylpyrrolidine P4 motifs 2y82 
Structure and property based design of factor Xa inhibitors: pyrrolidin-2-ones with aminoindane and phenylpyrrolidine P4 motifs 2zp0 
Human factor viia-tissue factor complexed with benzylsulfonamide-D-ile-gln-P-aminobenzamidine 2zwl 
Human factor viia-tissue factor complexed with highly selective peptide inhibitor 2zzu 
Human Factor VIIA-Tissue Factor Complexed with ethylsulfonamide-D-5-(3-carboxybenzyloxy)-Trp-Gln-p-aminobenzamidine 3bps 
PCSK9:EGF-A complex 3dem 
CUB1-EGF-CUB2 domain of HUMAN MASP-1/3 3ela 
Crystal structure of active site inhibited coagulation factor VIIA mutant in complex with soluble tissue factor 3ens 
Crystal structure of human FXA in complex with methyl (2Z)-3-[(3-chloro-1H-indol-7-yl)amino]-2-cyano-3-{[(3S)-2-oxo-1-(2-oxo-2-pyrrolidin-1-ylethyl)azepan-3-yl]amino}acrylate 3gcw 
PCSK9:EGFA(H306Y) 3gcx 
PCSK9:EGFA (pH 7.4) 3hpt 
Crystal structure of human FxA in complex with (S)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)azepan-3-yl)guanidine 3k9x 
X-ray crystal structure of human fxa in complex with (S)-N-((2-METHYLBENZOFURAN-5-YLAMINO)(2-OXO-1-(2-OXO-2- (PYRROLIDIN-1-YL)ETHYL)AZEPAN-3- YLAMINO)METHYLENE)NICOTINAMIDE 3m0c 
The X-ray Crystal Structure of PCSK9 in Complex with the LDL receptor 3p5b 
The structure of the LDLR/PCSK9 complex reveals the receptor in an extended conformation 3p5c 
The structure of the LDLR/PCSK9 complex reveals the receptor in an extended conformation 3sw2 
X-ray crystal structure of human FXA in complex with 6-chloro-N-((3S)-2-oxo-1-(2-oxo-2-((5S)-8-oxo-5,6-dihydro-1H-1,5-methanopyrido[1,2-a][1,5]diazocin-3(2H,4H,8H)-yl)ethyl)piperidin-3-yl)naphthalene-2-sulfonamide 3th2 
Mg2+ Is Required for Optimal Folding of the Gamma-Carboxyglutamic Acid (Gla) Domains of Vitamin K-Dependent Clotting Factors At Physiological Ca2+ 3th3 
Mg2+ Is Required for Optimal Folding of the Gamma-Carboxyglutamic Acid (Gla) Domains of Vitamin K-Dependent Clotting Factors At Physiological Ca2+ 3th4 
Mg2+ Is Required for Optimal Folding of the Gamma-Carboxyglutamic Acid (Gla) Domains of Vitamin K-Dependent Clotting Factors At Physiological Ca2+ 3v65 
Crystal structure of agrin and LRP4 complex 4a7i 
Factor Xa in complex with a potent 2-amino-ethane sulfonamide inhibitor 4aqb 
MBL-Ficolin Associated Protein-1, MAP-1 aka MAP44 4bti 
factor Xa in complex with the dual thrombin-FXa inhibitor 58. 4btt 
factor Xa in complex with the dual thrombin-FXa inhibitor 31. 4btu 
Factor Xa in complex with the dual thrombin-FXa inhibitor 57. 4bxs 
Crystal Structure of the Prothrombinase Complex from the Venom of Pseudonaja Textilis 4bxw 
Crystal Structure of the Prothrombinase Complex from the Venom of Pseudonaja Textilis 4cbz 
Notch ligand, Jagged-1, contains an N-terminal C2 domain 4cc0 
Notch ligand, Jagged-1, contains an N-terminal C2 domain 4cc1 
Notch ligand, Jagged-1, contains an N-terminal C2 domain 4cud 
Human Notch1 EGF domains 11-13 mutant fucosylated at T466 4cue 
Human Notch1 EGF domains 11-13 mutant T466V 4cuf 
Human Notch1 EGF domains 11-13 mutant T466S 4d0e 
Human Notch1 EGF domains 11-13 mutant GlcNAc-fucose disaccharide modified at T466 4d0f 
Human Notch1 EGF domains 11-13 mutant T466A 4d90 
Crystal Structure of Del-1 EGF domains 4ibl 
Rubidium Sites in Blood Coagulation Factor VIIa 4lmf 
C1s CUB1-EGF-CUB2 4lor 
C1s CUB1-EGF-CUB2 in complex with a collagen-like peptide from C1q 4wm0 
4WM0 4wma 
4WMA 4wmb 
4WMB 4wmi 
4WMI 4wmk 
4WMK 4wn2 
4WN2 4wnh 
4WNH 4xbm 
4XBM 4xl1 
4XL1 4xlw 
4XLW 4y6d 
4Y6D 4y71 
4Y71 4y76 
4Y76 4y79 
4Y79 4y7a 
4Y7A 4y7b 
4Y7B 4ylq 
4YLQ 4zh8 
4ZH8 4zha 
4ZHA 4zma 
4ZMA 5bo1 
5BO1 5f84 
5F84 5f85 
5F85 5f86 
5F86 5fm9 
5FM9 5fma 
5FMA - Links (links to other resources describing this domain)
-
PROSITE EGF_CA PFAM EGF INTERPRO IPR001881

